SARS-CoV-2 Antigen Assay Kit (Immunochromatography Method)
SARS-CoV-2 Antigen Assay Kit
(Immunochromatography Method) Product Manual
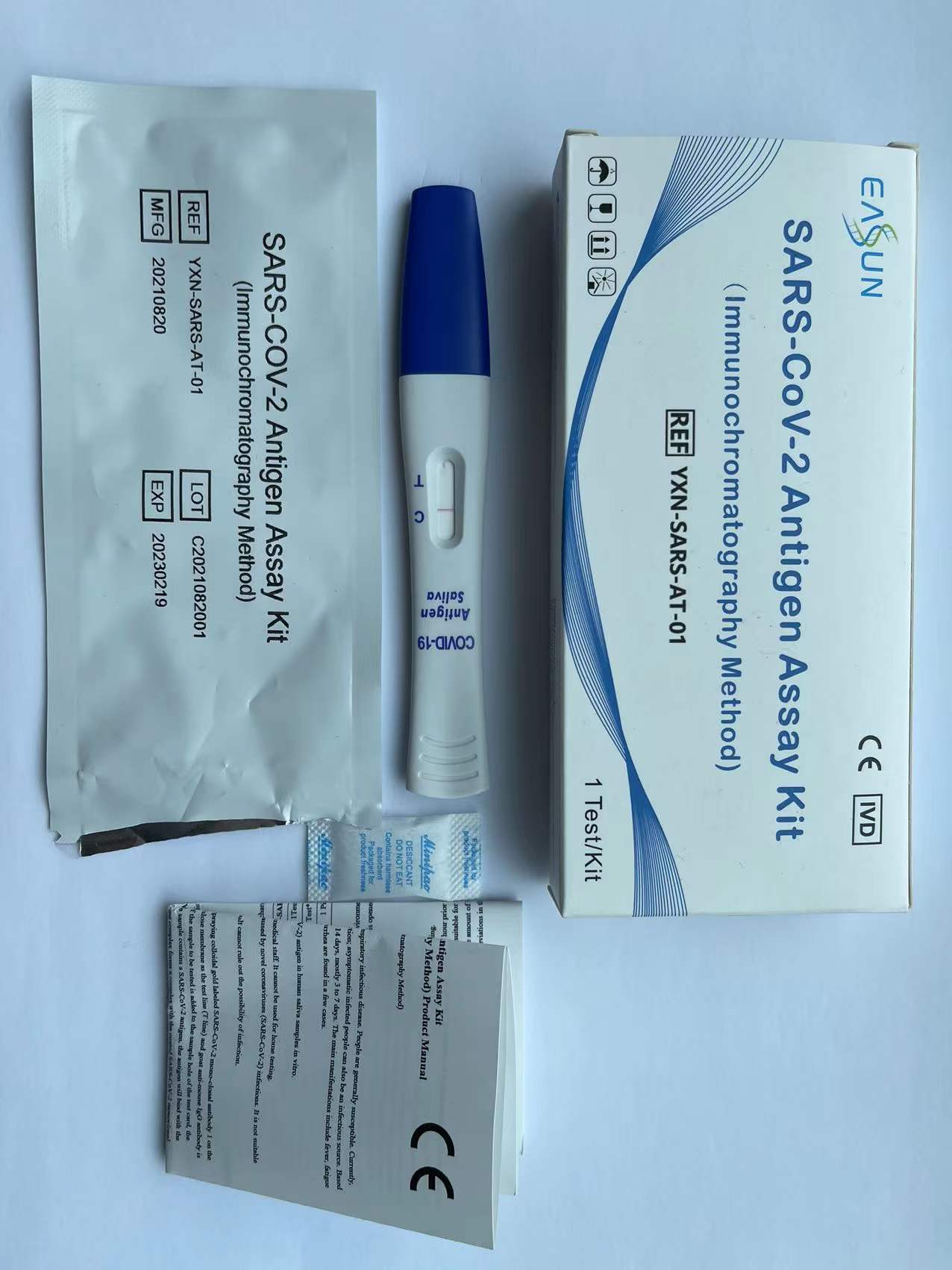
【PRODUCT NAME】SARS-CoV-2 Antigen Assay Kit (Immunochromatography Method)
【PACKAGING SPECIFICATIONS】 1 Test/Kit
【ABSTRACT】
The novel coronaviruses belong to the β genus. COVID- 19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
【EXPECTED USAGE】
This kit is used to qualitatively detect the novel coronaviruses (SARS-CoV-2) antigen in human saliva samples in vitro. It is only suitable for professional in vitro diagnosis, not for personal use.
This product is only used in clinical laboratories or immediate testing by medical staff. It cannot be used for home testing.
It cannot be used as the basis for diagnosis and exclusion of pneumonia caused by novel coronaviruses (SARS-CoV-2) infections. It is not suitable for screening by the general population.
A positive test result requires further confirmation, and a negative test result cannot rule out the possibility of infection.
【PRINCIPLES OF THE PROCEDURE】
This product adopts colloidal gold immunochromatography technology, spraying colloidal gold labeled SARS-CoV-2 mono-clonal antibody 1 on the gold pad The SARS-CoV-2 monoclonal antibody 2 is coated on nitrocellulose membrane as the test line (T line) and goat anti-mouse IgG antibody is coated as the quality control line (C line). When an appropriate amount of the sample to be tested is added to the sample hole of the test card, the sample will move forward along the test card under capillary action. If the sample contains a SARS-CoV-2 antigen, the antigen will bind with the colloidal gold labeled SARS-CoV-2 monoclonal antibody 1, and the immune complex forms a complex with the coated SARS-CoV-2 monoclonal antibody 2 at the T line, showing a purple-red T line, indicating that the SARS-CoV-2 antigen is positive. If the test line T does not show color and shows a negative result, it means that the sample does not contain the SARS-CoV-2 antigen. The test card also contains a quality control line C, regardless of whether there is a test line, the purple-red quality control line C should appear. If the quality control line C does not appear, it indicates that the test result is invalid, and this sample needs to be tested again.
【MAIN COMPONENTS】
( 1) Test card.
(2) Manual.
Note: The components in different batches of kits can’t be used interchangeably.
| Cat. No. | YXN-SARS-AT-01 |
| Package Specifications | 1Test/Kit |
| Test cassette | 1 Test* 1 pack |
| Manual | 1 Piece |
【STORAGE AND EXPIRATION】
The validity period is 18 months if this product is stored in an environment of 2℃-30℃.
The product should be used within 15 minutes once the foil bag is opened.Cover the lid immediately after taking out the sample extraction solution. The production date and expiration date are remarked on the label.
【SAMPLE REQUIREMENTS】
1. Applicable to human nasal throat swabs, oral throat swabs, saliva sample.
2. Sample collection:
( 1) Saliva collection(YXN-SARS-AT-01): Perform hand hygiene with soap and water /alcohol-based hand rub. Open the container. Make a Kruuua’ noise from the throat to clear the saliva from deep throat,then spit saliva (about 2 ml) into the container. Avoid any saliva contamination of the outer surface of the container. Optimal timing of specimen collection:After getting up and before brushing teeth, eating or drinking.
3. Process the sample immediately with sample extraction solution provided in the kit after the sample is collected. If it cannot be processed immediately, the sample should be stored in a dry, sterilized and strictly sealed plastic tube. It can be stored at 2℃ -8 ℃ for 8 hours , and can be stored for a long time at -70℃.
4. Samples that are heavily contaminated by oral food residues cannot be used for testing of this product. Samples collected from swabs that are too viscous or agglomerated are not recommended for testing of this product. If the swabs are contaminated with a large amount of blood, they are not recommended for testing. It is not recommended to use the samples that are processed with sample extraction solution not provided in this kit for testing of this product.
【TESTING METHOD】
Please read the instruction manual carefully before testing. Please return all reagents to room temperature before the test. The test should be performed at room temperature.
Test Steps:
1.Saliva sample(YXN-SARS-AT-01):
( 1) After the test cassette returns to room temperature, open the aluminum foil bag and take out the test cassette and place it horizontally on the desktop.
(2) Remove the tip of the test cassette, immerse the test cassette rod in saliva, or place the test cassette rod beneath tongue for 2 minutes.
(3) Keep the test cassette upright and let the saliva liquid move upward until it reach or move over Line C, then put back the lid and lay down the test cassette on the desk.
(4) Read the displayed results within 15-30 minutes, and the results read after 30 minutes are invalid.
【[INTERPRETATION OF TEST RESULTS】
| ★Both the test line (T) and the control line (C) show color bands as the picture shows as right, indicating that SARS-CoV-2 antigen is positive. | |
| ★NEGATIVE: If only the quality control line C develops color and the test line (T) does not develop color, the SARSCoV-2 antigen is not detected and the result is negative, as the picture shows as right. | |
| ★INVALID: No color band appears on the quality control line (C), and it is judged as an invalid result regardless of whether the detection line (T) shows color band or not, as the picture shows as right.Control line fails to appear.Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure.Review the procedure and repeat the test with a new test cassette.If the problem persist,discontinue using the test kit immediately and contact your local distributor. |
【LIMITATION OF DETECTION METHOD】
1. Clinical verification
In order to evaluate the diagnostic performance, this study used COVID- 19-positive specimens from 150 individuals and COVID- 19-negative specimens from 350 individuals. These specimens were tested and confirmed by the RT-PCR method. The results are as follows:
a) Sensitivity :92.67% (139/ 150), 95%CI (87.26% , 96.28%).
b) Specificity:98.29% ( 344/350), 95%CI( 96.31%, 99.37%) .
2. Minimum detection limit:
When the virus content is greater than 400TCID50/ml, the positive detection rate is greater than 95%. When the virus content is less than 200TCID50/ml, the positive detection rate is less than 95%, so the minimum detection limit of this product is 400TCID50/ml.
3. Precision:
Three consecutive batches of reagents were tested for precision. Different batches of reagents were used to test the same negative sample 10 times in succession, and the results were all negative. Different batches of reagents were used to test the same positive sample 10 times in succession, and the
results were all positive.
4. HOOK effect:
When the virus content in the sample to be tested reaches 4.0*105TCID50/ml, the test result still does not show the HOOK effect. 5. Cross-reactivity
Cross-reactivity of the Kit was evaluated. The results showed no cross reactivity with the following specimen.
| No | Item | Conc | No | Item | Conc |
| 1 | HCOV-HKU1 | 105TCID50/ml | 16 | Influenza A H3N2 | 105TCID50/ml |
| 2 | Staphylococcus aureus | 106TCID50/ml | 17 | H7N9 | 105TCID50/ml |
| 3 | Group A streptococci | 106TCID50/ml | 18 | H5N1 | 105TCID50/ml |
| 4 | Measles virus | 105TCID50/ml | 19 | Epstein-Barr virus | 105TCID50/ml |
| 5 | Mumps virus | 105TCID50/ml | 20 | Enterovirus CA16 | 105TCID50/ml |
| 6 | Adenovirus type 3 | 105TCID50/ml | 21 | Rhinovirus | 105TCID50/ml |
| 7 | Mycoplasmal pneumonia | 106TCID50/ml | 22 | Respiratory syncytial virus | 105TCID50/ml |
| 8 | Paraimfluenzavirus,type2 | 105TCID50/ml | 23 | Streptococcus pneumoniae | 106TCID50/ml |
| 9 | Human metapneumovirus | 105TCID50/ml | 24 | Candida albicans | 106TCID50/ml |
| 10 | Human coronavirus OC43 | 105TCID50/ml | 25 | Chlamydia pneumoniae | 106TCID50/ml |
| 11 | Human coronavirus 229E | 105TCID50/ml | 26 | Bordetella pertussis | 106TCID50/ml |
| 12 | Bordetella parapertusis | 106TCID50/ml | 27 | Pneumocystis jiroveci | 106TCID50/ml |
| 13 | Influenza B Victoria strain | 105TCID50/ml | 28 | Mycobacterium tubercu losis | 106TCID50/ml |
| 14 | Influenza B Y strain | 105TCID50/ml | 29 | Legionella pneumophila | 106TCID50/ml |
| 15 | Influenza A H1N1 2009 | 105TCID50/ml |
6. Interference Substances
The test results do not be interfeerd with the substance at the following concentration:
| No | Item | Conc | No | Item | Conc |
| 1 | Whole Blood | 4% | 9 | Mucin | 0 50% |
| 2 | Ibuprofen | 1mg/ml | 10 | Compound Benzoin Gel | 1.5mg/ml |
| 3 | tetracycline | 3ug/ml | 11 | Cromolyn glycate | 15% |
| 4 | chloramphenicol | 3ug/ml | 12 | Deoxyepinephrine hydro chloride | 15% |
| 5 | Erythromycin | 3ug/ml | 13 | Afrin | 15% |
| 6 | Tobramycin | 5% | 14 | Fluticasone propionate spray | 15% |
| 7 | Oseltamivir | 5mg/ml | 15 | menthol | 15% |
| 8 | Naphazoline Hydrochlo ride Nasal Drops | 15% | 16 | Mupirocin | 10mg/ml |
【LIMITATION OF DETECTION METHOD】
1. This product is only provided to clinical laboratories or medical staff for immediate testing, and cannot be used for home testing.
2. This product is only suitable for the detection of human nasal cavity or throat secretion samples. It detects the virus content in the sample extract, regardless of whether the virus is infectious. Therefore, the test results of this product and the virus culture results of the same sample may not be correlated.
3. The test card and sample extraction solution of this product need to be restored to room temperature before use. Improper temperature may cause abnormal test result.
4. During the testing process, the test results may not match the clinical results due to insufficient sample collection of sterile swabs or improper collection and specimen extraction operation.
5. During the use of this product, you need to strictly follow the operating steps of the manual. Improper operating steps and environmental conditions may cause abnormal test results.
6. The swab should be rotated about 10 times on the inner wall of the test tube containing the sample extraction solution. Too few or too many rotations may cause abnormal test results.
7. A positive result of this product cannot rule out the possibility of other pathogens being positive.
8. A negative test result f this product cannot rule out the possibility of other pathogens being positive.
9. Negative test results are recommended to be verified with nucleic acid detection reagents to avoid the risk of missed test.
10. There may be differences in test results between frozen clinical samples and freshly collected clinical samples.
11. The specimen should be tested immediately after collection to avoid abnormal test results after being left for too long.
12. During the use of this product, an appropriate sample amount is necessary, too little or too much sample amount may cause abnormal test results. It is recommended to use a pipette with a more accurate sample volume for sample addition test.
【PRECAUTIONS】
1. Please equilibrate the sample diluent and test card to room temperature (above 30min) before testing.
2. The inspection should be carried out in strict accordance with the instructions.
3. The result must be interpreted within 15-30min, and the result read after 30 min is invalid.
4. The test sample should be regarded as an infectious substance, and the operation must be carried out in accordance with the operating specifications of the infectious disease laboratory, with protective measures and attention to bio-safety operation.
5. This product contains animal-derived substances. Although it is not contagious, it should be treated with caution when handling potential sources of infection. Users should take protective measures to ensure the safety of themselves and others.
6. The used test cards, sample extracts, etc. are treated as bio-medical waste after the test, and wash your hands in time.
7. If the sample treatment solution of this product accidentally spills into the skin or eyes, please rinse immediately with plenty of water, and seek medical attention if necessary.
8. Do not use the kit with obvious damage, and test card with damaged package.
9. This product is a one-time use product, please do not reuse it, and do not use expired products.
10. Avoid direct sunlight and direct blowing from electric fans during testing.
11. Tap water, distilled water or deionized water and beverages cannot be used as negative control reagents.
12. Due to the difference of the samples, some test lines may be lighter or grayish in color. As a qualitative product, as long as there is a band at the position of the T line, it can be judged as positive.
13. If the test is positive, it is recommended to use this test card to recheck once to avoid small probability events.
14. There is a desiccant in the aluminum foil bag, do not take it orally