COVID-19 IgM/IgG Antibody Detection Kit
COVID-19 IgM/IgG Antibody Detection Kit
(Colloidal Gold Immunochromatography Method) Product Manual
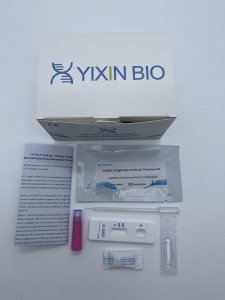
【PRODUCT NAME】COVID- 19 IgM/IgG Antibody Detection Kit (Colloidal Gold Immunochromatography Method) 【PACKAGING SPECIFICATIONS】 1 Tests/Kit ,10 Tests/Kit
【ABSTRACT】
The novel coronaviruses belong to the β genus. COVID- 19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
【EXPECTED USAGE】
This kit is suitable for the qualitative detection of COVID- 19 by detecting 2019- nCoV IgM/IgG antibodies in human serum, plasma, or whole blood. Common signs of infection with 2019-nCoV include respiratory symptoms, fever, cough, shortness of breath, and dyspnea. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death. 2019 nCoV can be excreted through respiratory secretions or transmitted through oral fluids, sneezing, physical contact, and through air droplets.
【PRINCIPLES OF THE PROCEDURE】
The principle of immunochromatography of this kit: the separation of components in a mixture through a medium using capillary force and the specific and rapid binding of an antibody to its antigen. This test consists of two cassettes, an IgG cassette and an IgM cassette.
For YXI-CoV- IgM&IgG- 1 and YXI-CoV- IgM&IgG- 10:In the IgM cassette, it is a dry medium that has been coated separately with 2019-nCoV recombinant antigen ( “T” test line) and goat anti-mouse polyclonal antibodies ( “C” control line). The colloidal gold-labeled antibodies, mouse anti-human IgM (mIgM) is in the release pad section.Once diluted serum, plasma, or whole blood is applied to the sample pad section(S), the mIgM antibody will bind to 2019-nCoV IgM antibodies if they are present, forming an mIgM-IgM complex. The mIgM-IgM complex will then move across the nitrocellulose filter(NC filter) via capillary action. If 2019-nCoV IgM antibody is present in the sample, the test line (T) will be bound by the mIgM-IgM complex and develop color. If there is no 2019-nCoV IgM antibody in the sample, free mIgM will not bind to the test line (T) and no color will develop. The free mIgM will bind to the control line (C); this control line should be visible after the detection step as this confirms that the kit is working properly.In the IgG cassette, it is a dry medium that has been coated separately with mouse anti-human IgG ( “T” test line) and Rabbit antichicken IgY antibody ( “C” control line). The colloidal gold-labeled antibodies, 2019-nCoV recombinant antigen and chicken IgY antibody are in the release pad section. Once diluted serum, plasma, or whole blood is applied to the sample pad section(S), the
colloidalgold-2019-nCoV recombinant antigen will bind to 2019-nCoV IgG antibodies if they are present, forming a colloidalgold-2019-nCoV recombinant antigen-IgG complex. The complex will then move across the nitrocellulose filter (NC filter) via capillary action. If 2019-nCoV IgG antibody is present in the sample, the test line (T) will be bound by the colloidalgold-2019-nCoV recombinant antigen-IgG complex and develop color. If there is no 2019-nCoV IgG antibody in the sample, free colloidalgold-2019-nCoV recombinant antigen will not bind to the test line (T) and no color will develop. The free colloidal gold-chicken IgY antibody will bind to the control line (C); this control line should be visible after the detection step as this confirms that the kit is working properly.
For For YXI-CoV- IgM&IgG-02- 1 and YXI-CoV- IgM&IgG-02- 10:The principle of immunochromatography of this kit: the separation of components in a mixture through a medium using capillary force and the specific and rapid binding of an antibody to its antigen. The COVID- 19 IgM/IgG Antibody Detection Kit is a qualitative membrane-based immunoassay for the detection of IgG and IgM antibodies to SARS-CoV-2 in whole blood, serum or plasma specimens. This test consists of two components, an IgG component and an IgM component. In the IgG component, anti-human IgG is coated in the IgG test line region. During testing, the specimen reacts with SARS-CoV-2 antigen-coated particles in the test cassette. The mixture then migrates laterally along the membrane chromatographically by capillary action and reacts with the antihuman IgG in the IgG test line region, if the specimen contains IgG antibodies to SARSCoV-2. A coloured line will appear in the IgG test line region as a result of this. Similarly, anti-human IgM is coated in the IgM test line region and if the specimen contains IgM antibodies to SARS-CoV-2, the conjugate specimen complex reacts with antihuman IgM. A coloured line appears in the IgM test line region as a result. Therefore, if the specimen contains SARS-CoV-2 IgG antibodies, a coloured line will appear in the IgG test line region. If the specimen contains SARS-CoV-2 IgM antibodies, a coloured line will appear in the IgM test line region. If the specimen does not contain SARS-CoV-2 antibodies, no coloured line will appear in either of the test line regions, indicating a negative result. To serve as a procedural control, a coloured line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
【MAIN COMPONENTS】
| Cat. No. | YXI-CoV-IgM&IgG-1 | YXI-CoV-IgM&IgG-10 | YXI-CoV-IgM&IgG-02-1 | YXI-CoV-IgM&IgG-02-10 |
Components |
|
|
Product Pic. |
||||||
| Name | Specification | Quantity | Quantity | Quantity | Quantity | |
| test strip type 1 | 1 test/bag | / | / | 1 | 10 | Nitrocellulose membrane, binding pad, sample pad, blood filtration membrane, absorbent paper, PVC |
| test strip type 2 | 1 test/bag | 1 | 10 | / | / | Nitrocellulose membrane, binding pad, sample pad, blood filtration membrane, absorbent paper, PVC |
| sample diluent tube | 100 μL/vial | 1 | 10 | 1 | 10 | Phosphate, Tween-20 |
| desiccant | 1 piece | 1 | 10 | 1 | 10 | silicon dioxide |
| dropper | 1 piece | 1 | 10 | 1 | 10 | Plastic |
Note : The components in different batch kits cannot be mixed or interchanged .
【MATERIALS TO BE PROVIDED BY USER】
•Alcohol pad
•Blood taking needle
【STORAGE AND EXPIRATION】
Keep kits in a cool and dry place at 2 - 25°C.
Do not freeze.
Properly stored kits are valid for 12 months.
【SAMPLE REQUIREMENTS】
Assay is suitable for human serum, plasma, or whole blood samples. Samples should be used as soon as possible after collection. Serum and plasma collection: Serum and plasma should be separated as soon as possible after blood collection to avoid hemolysis.
【SAMPLE PRESERVATION】
Serum and plasma should be used as soon as possible after collection and stored at 2-8°C for 7 days if not use immediately. If long-term storage is required, please store at -20 °C for periods less than 2 months. Avoid repeated freezing and thawing.
Whole or peripheral blood sample should be tested within 8 hours after collection.
Severe hemolysis and lipid blood samples shall not be used for detection.
【TESTING METHOD】
For YXI-CoV- IgM&IgG- 1 and YXI-CoV- IgM&IgG- 10:
Read the instructions carefully before use. Bring the Test strip, Sample diluent tube, and sample to room temperature before testing.
1. Add 50 µl of Whole or peripheral blood or 20 µl Serum and plasma to the Sample diluent tube and mix thoroughly. Add 3- 4 drops to the sample pad section.
2. Leave at room temperature for 5 minutes to observe the results. Results measured after 5 minutes are invalid and should be discarded. For YXI-CoV- IgM&IgG-02- 1 and YXI-CoV- IgM&IgG-02- 10:
Read the instructions carefully before use. Bring the Test strip, Sample diluent tube, and sample to room temperature before testing.
1. Add 25µl of Whole or peripheral blood or 10µl Serum and plasma to the Sample diluent tube and mix thoroughly. Add 4 drops to the sample pad
section.
2. Leave at room temperature for 5 minutes to observe the results. Results measured after 5 minutes are invalid and should be discarded.
【[INTERPRETATION OF TEST RESULTS 】
| YXI-CoV- IgM&IgG-1 and YXI-CoV- IgM&IgG-10 | YXI-CoV- IgM&IgG-02-1 and YXI-CoV- IgM&IgG-02-10 |
| ★IgG POSITIVE: Two lines appear.One coloured line should be in the control line region (C),and a coloured line appears in the IgG test line region.The result is positive for 2019- nCoV specific-IgG antibodies. ★lgM POSITIVE: Two lines appear. One coloured line should be in the control line region(C), and a coloured line appears in the lgM test line region.The result is positive for 2019- nCoV specific-lgM antibodies.★IgG and lgM POSITIVE: Both the test line (T)and the quality control line (C)are colored in an IgG cassette and an lgM cassette.
★NEGATIVE: One coloured lie appears in the control region (C).No apparent coloured line appears in the lgG or lgM test region(T).
★INVALID: Control line fails to appear.Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure.Review the procedure and repeat the test with a new test cassette.If the problem persist,discontinue using the test kit immediately and contact your local distributor.
|
★IgG POSITIVE: Two lines appear. One coloured line should be in the control line region (C), and a coloured line appears in the IgG test line region. The result is positive for SARS-CoV-2 specific-IgG antibodies. ★IgM POSITIVE: Two lines appear. One coloured line should be in the control line region (C), and a coloured line appears in the IgM test line region. The result is positive for SARS-CoV-2 specific-IgM antibodies. ★IgG and IgM POSITIVE: Three lines appear. One coloured line should be in the control line region (C), and two coloured lines should appear in IgG test line region and IgM test line region.
★NEGATIVE: One coloured line appears in the control region (C). No apparent coloured line appears in the IgG or IgM test region (T).
★INVALID: Control line fails to appear. Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette.If the problem persists, discontinue using the test kit immediately andcontactyour local distributor.
|
【LIMITATION OF DETECTION METHOD】
a. The product is designed only for use with human serum, plasma, whole blood samples for the qualitative detection of 2019 -nCoV IgM and IgG antibody.
b. As in case of all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test but should rather be made after all the clinical findings have been evaluated and should be confirmed by other conventional detection methods.
c. A false negative may occur if the amount of 2019-nCoV IgM or IgG antibody is below the detection level of the kit.
d. If the product gets wet prior to use, or is stored improperly, it may cause incorrect results.
e. The test is for qualitative detection of 2019-nCoV IgM or IgG antibody in human serum, plasma or blood sample and does not indicate the quantity of the antibodies.
【PRECAUTIONS】
a. Do not use expired or damaged products.
b. Only use the matching diluent in the kit package. Diluents from different kit lots cannot be mixed.
c. Do not use tap water, purified water or distilled water as negative controls.
d. The test should be used within 1 hour after opening. If the ambient temperature is higher than 30 ℃, or the test environment is humid, the Detection Cassette should be used immediately.
e. If there is no movement of the liquid after 30 seconds of beginning the test, additional drop of sample solution should be added.
f. Take care to prevent the possibility of virus infection when collecting samples. Wear disposable gloves, masks, etc., and wash your hands afterwards.
g. This test card is designed for a single, one-time use. After use, the test card and samples should be regarded as medical waste with risk of biological infection and properly disposed of in accordance with relevant national regulations .